What is Tamoxifen? Research Information
Product information
- formula – C32H37NO8
- Molecular weight – 563.6 g/mol
- Bioavailability – approximately 100%
- – 5-7 days
Other names:
- Istubal
- Kessar
- Tamoxifen (Citrate)
- Zitazonium
- Zemide
- Tamoxifen citrate salt
- UNII-7FRV7310N6
- ICI 46474
- C32H37NO8
- Tamoxifen Citrate (Nolvadex)
- ICI 46474 citrate
- ICI 46,474
- 7FRV7310N6
- 54965-24-1 (citrate)
- NSC180973
- MFCD00058321
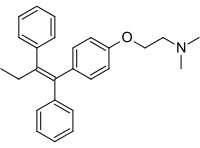
Discovery of tamoxifen citrate:
In 1962, tamoxifen was first discovered by British scientists at a British company known as Imperial Chemical Industries (which is now known as AstraZeneca). Initially, Dora Richardson, a scientist, made tamoxifen.
At first, tamoxifen was used as a contraceptive drug due to its effects in rats via the antioestrogen mechanism. In the early 1970s, tamoxifen showed the opposite effect in humans by increasing the fertility of women via ovulation stimulation. Now, tamoxifen is the most used anti-cancer drug, which is also useful for other conditions such as infertility, prostate cancer, gynecomastia, and other anovulatory disorders.
Mechanism of action:
Tamoxifen tends to have a dual mechanism of action.
At first, tamoxifen competes with the E2 at the receptors site (17-beta oestradiol) and blocks the E2 promotional role in the development of breast cancer.
Secondly, tamoxifen binds to the DNA after the activation of a metabolic pathway for carcinogenesis initiation.
Tamoxifen belongs to the antioestrogen medication class. It tends to block the activity of the female hormone (estrogen) in the breasts. It can also help prevent the growth of other types of tumors dependent on estrogen for their growth.
Clinical applications:
Metastatic breast cancer treatment:
Tamoxifen citrate is effective for the treatment of metastatic breast cancer in patients. In postmenopausal women, tamoxifen citrate acts as an alternative for ovarian irradiation or oophorectomy. According to the clinical evidence, patients with positive estrogen receptor tumors have more chances of a good prognosis with tamoxifen citrate.
Adjuvant treatment for breast cancer:
Tamoxifen citrate indicates the treatment of breast cancer in women (node-positive breast cancer) after breast irradiation, total mastectomy, segmental mastectomy, and axillary dissection. Adjuvant treatment with tamoxifen citrate helps reduce contralateral breast cancer in patients with breast cancer.
Prevention of invasive breast cancer:
In women suffering from ductal carcinoma in situ (DCIS), tamoxifen citrate is indicated for reducing the risk of invasive breast cancers following radiation and breast surgery. Most of the time, tamoxifen citrate is indicated for patients (females) at a higher risk of cancer development.
Disclaimer
The information provided about Tamoxifen Citrate in this section is only for research advancement and disbursement of knowledge. The material collected in this article is meant for informational purposes about Tamoxifen . It is not to be considered instructional in any way. Moreover, we have further empathized with this by making sure no dosage information of Tamoxifen or recommendations about its way of consumption is mentioned. The information available in this article is a collection from different recognized studies and researches conducted by known experts and researchers in controlled medical facilities and institutions. Furthermore, the information provided in the article is not to encourage the reader to start its consumption or as an advertisement of the product. Administering any supplement or medication not FDA approved may be harmful and may cause serious illness. Peptide Pros insist that none of their products be ingested under any circumstances.
References:
- Tamoxifen citrate | Compound| Pubchem| https://pubchem.ncbi.nlm.nih.gov/compound/Tamoxifen-citrate
- Tamoxifen citrate | Compound|Chemspider| http://www.chemspider.com/Chemical-Structure.2015313.html
- Love, R. R. (1989). Tamoxifen therapy in primary breast cancer: biology, efficacy, and side effects. Journal of clinical oncology, 7(6), 803-815.
- Plotkin, D., Lechner, J. J., Jung, W. E., & Rosen, P. J. (1978). Tamoxifen flare in advanced breast cancer. Jama, 240(24), 2644-2646.
- Maji, R., Dey, N. S., Satapathy, B. S., Mukherjee, B., & Mondal, S. (2014). Preparation and characterization of Tamoxifen citrate loaded nanoparticles for breast cancer therapy. International journal of nanomedicine, 9, 3107.
- Osborne, C. K. (1998). Tamoxifen in the treatment of breast cancer. New England Journal of Medicine, 339(22), 1609-1618.
- Daly, Mary B. “Tamoxifen in ductal carcinoma in situ.” Seminars in oncology. Vol. 33. No. 6. WB Saunders, 2006.
Where to find Tamoxifen for sale?
If you’re looking to buy Tamoxifen 20 mg for research purposes, shop at Peptide Pros, the most reliable supplier of the highest quality SARMS and USA peptides for sale: https://www.peptidepros.net/product/tamoxifen-citrate-20mcg-ml-30ml-bottle/
 verified reviews, and counting
verified reviews, and counting
